Abstract
Background: We have previously published our observations regarding targeted next generation sequencing (NGS) in primary myelofibrosis (PMF), essential thrombocythemia (ET) and polycythemia vera (PV) (Blood Advances 2016 1:105; Blood Advances 2016 1:21). In the current study, we took a similar approach in order to examine the mutational repertoire in blast phase myeloproliferative neoplasms (MPN-BP) and explore its clinical correlates, including phenotype, treatment response and survival.
Methods:
Diagnoses of MPN, including PMF, PV, ET and MPN-BP were according to WHO criteria (Blood 2016;127:2391). The study population was selected on the basis of the availability of sufficient DNA and was not otherwise biased by any other selection criteria. Targeted capture assays were carried out on bone marrow or whole blood DNA, according to previously published methods (Blood Advances 2016 1:105). Statistical analyses considered clinical and laboratory data collected at the time of blast transformation. Survival was calculated from the date of leukemic transformation to the date of death or last contact. Treatment response in MPN-BP was assessed according to conventional criteria (JCO 2003;21:4642).
Results:
72 consecutive cases of MPN-BP underwent target capture NGS using a panel of 39 genes.
Mutational landscape, frequencies and co-segregation patterns
Driver mutations were detected in 62 (86%) patients, including JAK2 only in 39 (54%), JAK2 + CALR 3 (4%), JAK2 + MPL 3 (4%), CALR only 10 (14%), CALR + MPL 2 (3%) and MPL only 5 (7%). Sixty-six (92%) patients displayed at least one mutation/DNA variant, other than JAK2 / CALR / MPL ; the number (%) of patients with 0, 1, 2, 3, 4, 5, 6 or 7 such mutations was 6 (8%), 9 (13%), 19 (26%), 19 (26%), 13 (18%), 5 (7%), 0 and 1 (1.4%). The most frequent mutations (incidence), other than JAK2 / CALR / MPL were ASXL1 (46%), RUNX1 (22%), TET2 (21%), TP53 (17%), EZH2 (15%), IDH1 (14%), SRSF2 (14%), FLT3 (13%), and SH2B3 (11%). JAK2 mutations clustered with ASXL1 (p=0.02); MPL with IDH1 (p=0.0004) and SF3B1 (p=0.008); ASXL1 with EZH2 (p=0.009); RUNX1 with SRSF2 (p=0.02) and SH2B3 (p=0.04); SRSF2 with IDH2 (p=0.002); IDH2 with SH2B3 (p=0.04); and EZH2 with DNMT3A (p=0.0007).
Clinical course and management
At the time of this writing, complete clinical and follow-up data were available in 66 patients with MPN-BP (median age 65 years; 65% males). Induction chemotherapy for MPN-BP was given to 38 patients, resulting in complete remission (CR) or CR with incomplete recovery of counts (CRi) in 14 (37%) patients. Treatment for MPN-BP included allogenic stem cell transplant (ASCT) in 6 patients. Median follow-up from time of leukemic transformation was 3.9 months with 64 documented deaths (97%).
Impact of mutations on survival and treatment response rates
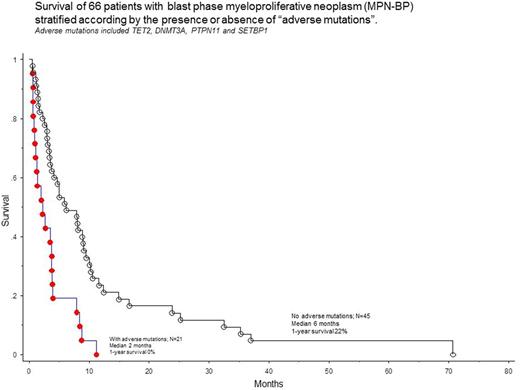
In univariate analysis, adverse impact on survival was demonstrated for TET2 (p=0.03), DNMT3A (p=0.05), PTPN11 (p=0.008) and SETBP1 (p=0.01) mutations, which were subsequently grouped as "adverse mutations". Twenty-one (32%) patients harbored at least one adverse mutation. Patients with or without adverse mutations were similar in terms of their age and gender distribution, clinical characteristics at time of leukemic transformation and karyotype (P>0.05 in all instances). Spectrum and frequency of adverse mutations were similar among specific driver mutations, including triple-negative cases (p=0.97).
The presence of adverse mutations was strongly predictive of shortened survival (HR 2.8, 95% CI 1.6-5.0; p=0.0003; Figure 1) and the effect was independent of several other clinical and laboratory variables including age, karyotype, specific MPN-BP morphologic category, blast burden and treatment undertaken for MPN-BP. In multivariable analysis, survival was independently affected by presence of adverse mutations (HR 4.8, 95% CI 2.0-11.8) and achievement of CR/CRi (HR 0.4; 95% CI 0.2-0.8), but not by ASCT. The presence of adverse mutations did not affect response rate to induction chemotherapy (p=0.6).
Conclusions:
The current study identifies the presence of prognostically-adverse mutations in approximately a third of patients with blast phase MPN (MPN-BP). The survival effect of adverse mutations was independent of other disease features and treatment strategies, including whether or not patients achieved CR/CRi or received ASCT.
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal